Magic fingers art



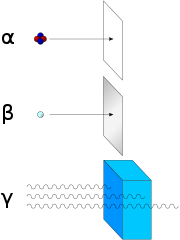
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay (𝛼-decay), beta decay (𝛽-decay), and gamma decay (𝛾-decay), all of which involve emitting one or more particles. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetic and strong forces.
Radioactive decay is a stochastic (i.e. random) process at the level of single atoms. According to quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant or as half-life. The half-lives of radioactive atoms have a huge range; from nearly instantaneous to far longer than the age of the universe.
The decaying nucleus is called the parent radionuclide , and the process produces at least one daughter nuclide. Except for gamma decay or internal conversion from a nuclear excited state, the decay is a nuclear transmutation resulting in a daughter containing a different number of protons or neutrons (or both). When the number of protons changes, an atom of a different chemical element is created.

Radioactivity was discovered in 1896 by scientists Henri Becquerel and Marie Curie, while working with phosphorescent materials. These materials glow in the dark after exposure to light, and he suspected that the glow produced in cathode ray tubes by X-rays might be associated with phosphorescence. He wrapped a photographic plate in black paper and placed various phosphorescent salts on it. All results were negative until he used uranium salts. The uranium salts caused a blackening of the plate in spite of the plate being wrapped in black paper. These radiations were given the name "Becquerel Rays".
It soon became clear that the blackening of the plate had nothing to do with phosphorescence, as the blackening was also produced by non-phosphorescent salts of uranium and by metallic uranium. It became clear from these experiments that there was a form of invisible radiation that could pass through paper and was causing the plate to react as if exposed to light.
At first, it seemed as though the new radiation was similar to the then recently discovered X-rays. Further research by Becquerel, Ernest Rutherford, Paul Villard, Pierre Curie, Marie Curie, and others showed that this form of radioactivity was significantly more complicated. Rutherford was the first to realize that all such elements decay in accordance with the same mathematical exponential formula. Rutherford and his student Frederick Soddy were the first to realize that many decay processes resulted in the transmutation of one element to another. Subsequently, the radioactive displacement law of Fajans and Soddy was formulated to describe the products of alpha and beta decay.
The early researchers also discovered that many other chemical elements, besides uranium, have radioactive isotopes. A systematic search for the total radioactivity in uranium ores also guided Pierre and Marie Curie to isolate two new elements: polonium and radium. Except for the radioactivity of radium, the chemical similarity of radium to barium made these two elements difficult to distinguish.
Marie and Pierre Curie's study of radioactivity is an important factor in science and medicine. After their research on Becquerel's rays led them to the discovery of both radium and polonium, they coined the term "radioactivity" to define the emission of ionizing radiation by some heavy elements. Their research on the penetrating rays in uranium and the discovery of radium launched an era of using radium for the treatment of cancer. Their exploration of radium could be seen as the first peaceful use of nuclear energy and the start of modern nuclear medicine.
Decay modes | |||
|---|---|---|---|
| Mode of decay | Participating particles | Daughter nucleus | |
| Decays with emission of nucleons | |||
| α | Alpha decay | An alpha particle (A = 4, Z = 2) emitted from nucleus | (A − 4, Z − 2) |
| p | Proton emission | A proton ejected from nucleus | (A − 1, Z − 1) |
| 2p | Double proton emission | Two protons ejected from nucleus simultaneously | (A − 2, Z − 2) |
| n | Neutron emission | A neutron ejected from nucleus | (A − 1, Z) |
| 2n | Double neutron emission | Two neutrons ejected from nucleus simultaneously | (A − 2, Z) |
| SF | Spontaneous fission | Nucleus disintegrates into two or more smaller nuclei and other particles | — |
| CD | Cluster decay | Nucleus emits a specific type of smaller nucleus (A1, Z1) which is larger than an alpha particle | (A − A1, Z − Z1) + (A1, Z1) |
| Different modes of beta decay | |||
| β− | Beta minus decay | A nucleus emits an electron and an electron antineutrino | (A, Z + 1) |
| β+ | Beta plus decay | A nucleus emits a positron and an electron neutrino | (A, Z − 1) |
| ε (EC) | Electron capture | A nucleus captures an orbiting electron and emits a neutrino; the daughter nucleus is left in an excited unstable state | (A, Z − 1) |
| Bound-state beta decay | A free neutron or nucleus beta decays to electron and antineutrino, but the electron is not emitted, as it is captured into an empty K-shell; the daughter nucleus is left in an excited and unstable state. This process is a minority of free neutron decays (0.0004%) due to the low energy of hydrogen ionization, and is suppressed except in ionized atoms that have K-shell vacancies. | (A, Z + 1) | |
| β−β− | Double beta decay | A nucleus emits two electrons and two antineutrinos | (A, Z + 2) |
| εε | Double electron capture | A nucleus absorbs two orbital electrons and emits two neutrinos – the daughter nucleus is left in an excited and unstable state | (A, Z − 2) |
| Electron capture with positron emission | A nucleus absorbs one orbital electron, emits one positron and two neutrinos | (A, Z − 2) | |
| β+β+ | Double positron decay | A nucleus emits two positrons and two neutrinos | (A, Z − 2) |
| Transitions between states of the same nucleus | |||
| IT | Isomeric transition | Excited nucleus releases a high-energy photon (gamma ray) | (A, Z) |
| Internal conversion | Excited nucleus transfers energy to an orbital electron, which is subsequently ejected from the atom | (A, Z) | |
Comments
Post a Comment